iO:M8
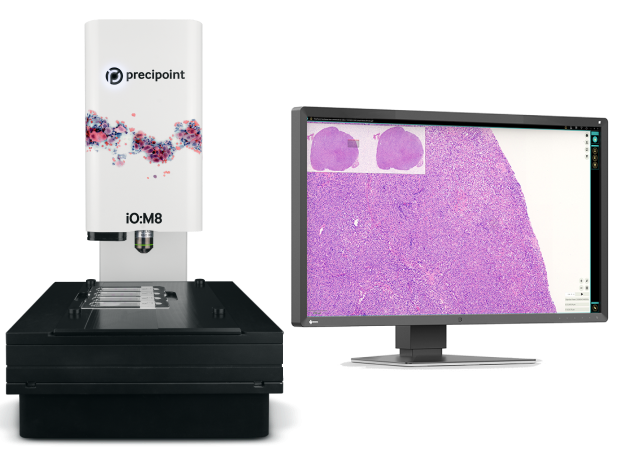
Diagnose digitally.
The digital microscope, for the highest quality and efficiency in frozen sections / ROSE.
A device with a live access feature to frozen sections.
With the iO:M8 digital live microscope, there is no need to transport samples physically. Now diagnose intraoperative cases reliably, efficiently, and remotely. Your work is digitized and simplified, and you no longer need to scan slides. You save on waiting time and avoid rescanning whole slide images (WSI).
The iO:M8 allows you to effortlessly navigate the most complex samples in real-time. Unsurpassed image quality, easy handling, and intuitive focusing let you work as efficiently and flexibly as with a traditional microscope.
By loading the video, you agree to YouTube's privacy policy.
Learn more
Robotic live microscopy at your fingertips
The IO:M8 is a fully motorized digital microscope for on-site rapid microscopic assessments.

Intraoperative consultations

Frozen sections, Mohs, FNA and more
High-resolution, real-time imaging

Access second opinions
Experience distinct hands-on robotic microscopy
The digital microscope delivers live images of amazing quality. Examine even very complex specimens live on your screen. Navigate seamlessly through the slide in real-time, zoom and refocus on any region of interest, as you would with your conventional microscope.
Control the microscope on your screen and examine the microscopic sample in real-time.
Hands-On, Live, Seamless
Intraoperative consultations are time-critical. With the iO:M8, the slides are available instantly for rapid microscopic assessment. As a healthcare professional, you can evaluate the live microscopic images on your screen right away, without having to wait for a Whole Slide Image (WSI)

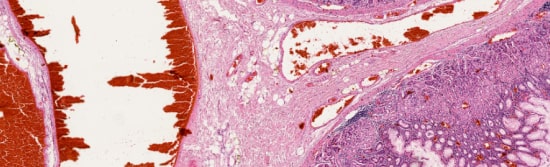
The iO:M8 always displays a preview image for easy orientation. Intuitively navigate to a region of interest, zoom in and refocus seamlessly down to the details of a live microscopic image, on your screen.
With the iO:M8, there is no need to wsi-scan slides. Not only do you save waiting time, but you also avoid rescanning low-quality wsi-images resulting from complex and uneven specimens such as smears, and sections with ice crystal or other artifacts as commonly found in frozen sections.
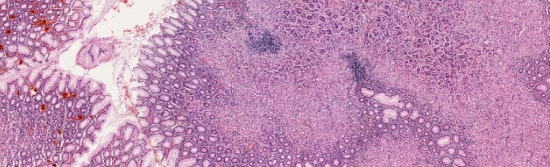
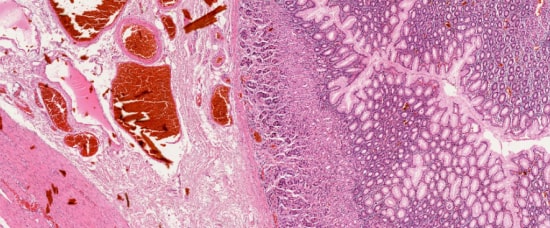
The rapid microscopic interpretation of intraoperative pathology slides can be very tricky. Get access to a second opinion from a specialist by simply granting them access to the on-site computer via the PreciPoint Streaming Software.
As a technical assistant, all you need to do is lay the glass slides under the microscope and start the device. The open x-y stage provides easy and direct access to the slides. Changing slides and cleaning is easy, unlike closed-box systems, where leaking mounting medium may be very hard to clean.
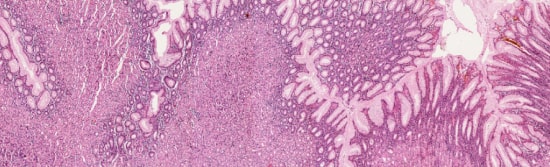
This is real digital microscopy
Shortest Time to View
The iO:M8 displays the slides in real-time on your screen for instant live microscopy.
Continuous Zoom and Focus
Start from the slide preview to find ROIs and seamlessly zoom in and refocus.
High-Resolution
Live images with the amazing high quality, colour and contrast you’ve come to expect from PreciPoint.

Robotic Microscopy
The image is completely in your control. Use the microscopy software to steer the fully motorized x-y stage and z- axis.
Easy Loading
Insert up to four standard slides onto the open, direct-access x-y stage for immediate viewing in one go.
Fast Set Up
Unpack and get started. No integration into LIMS or IT infrastructure necessary.

Download iO:M8 Brochure
Your privacy is important to us. PreciPoint uses your information to contact you about relevant content, products and services.
You can unsubscribe from any communication at any time via the footer of our emails. You can find more information in our data privacy policy.
Would you like to learn more?
Contact us to discuss how we can work together to digitize your intraoperative microscopy workflows.
*iO:M8 device is CE-IVD certified in conformity with Regulation (EU) 2017/746 of the European Parliament and of the Council of 05. April 2017 on in vitro diagnostic medical devices. The iO:M8 is registered in conformity with FDA regulation.




